Historical Background
The Kallikrein-Kinin System

Emil-Karl Frey, Eugen Werle, and Heinrich Kraut (left to right)
The surgeon Emil-Karl Frey, a scholar of the famous ‘Geheimrat’ Ferdinand Sauerbruch, observed in 1925 a considerable reduction in arterial blood pressure when he injected human urine into dogs. Unlike many other contemporary scientists he did not attribute this effect to a toxic action of urine, but rather as the specific activity of an unknown substance with potential biological functions (Frey, 1926; Frey and Kraut, 1926): “It is a substance that probably originates from several organs, is eliminated by the kindneys and has a pronounced cardioactive and vasoactive effect: a substance that is assigned the role of a hormone in the organism”. This F-substance was then called kallikrein, since it was considered to have originated in the pancreas (Greek synonym: kallikreas) (Kraut et al, 1930).
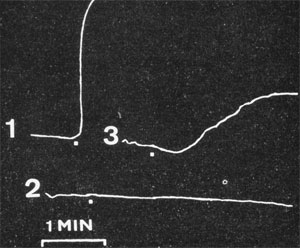
Kallidin liberation (1) by kallikrein and inactivation
in serum (2) as measured by muscle strip constriction
(Werle et al., Biochem Z. 289: 217, 1937)
Ten years later, Eugen Werle (Werle et al., 1937) identified kallikrein as a proteolytic enzyme (‘ferment’) that liberates the biologically highly active, basic polypeptide ‘DK’ or kallidin (i.e lys-bradykinin) from a blood plasma protein called kallidinogen or kininogen (H- and L-kininogen). Hence, kallidin was the first of the basic peptides formed by proteolysis to be identified as a hormone, and later became known as lys-bradykinin, a member of the family of biologically active kinin peptides. Kallidin was first characterized in great detail, especially with regard to its manifold pharmacological effects (Frey et al., 1950). Werle also observed for the first time irreversible ‘fermental degradation’ of kallidin by ‘kininases’ and identified them as peptidases (Werle and Grunz, 1939). Therefore, the fundamental knowledge of the system that we refer to today as the kallikrein-kinin system (cascade) was provided by Frey, Kraut and Werle.
The vital importance of the kallikrein-kinin system for basic mechanisms in biochemistry, patho/physiology, pharmacology and more recently, molecular biology and cell biology, and the great interest and practical benefit of the system to clinical medicine, has stimulated scientists from various disciplines worldwide to become involved in kallikrein-kinin research. Among them are scientists working also on various regulatory or mediator systems that interact with the kallikrein-kinin cascade. In a special highlight issue of Biological Chemistry (Vol. 382, pp. 3-139, 2001) leading experts in their field reviewed relevant topics of major interest, especially regarding (i) the regulation or function of kinin receptors and intracellular signalling events, (ii) the regulatory or therapeutic potential of kinin receptor antagonists in biology, pharmacology and medicine, and (iii) special cellular events associated with the kallikrein-kinin system.
The founder Henning L. Voigt,
his son Jason and his wife Garcia
In view of the present political and economic pressure to produce applicable scientific results in a minimal period of time, it is remarkable to emphasize that only recently, i.e. nearly 70 years after the discovery of the kininases, a drug based on the seminal research following their discovery and designated as angiotensin I converting enzyme (ACE) inhibitor has been shown to be of immense value in the treatment of coronary heart disease (HOPE Study Investigators, 1996; The Heart Outcome Prevention Evaluation Study Investigators, 2000; Results of the HOPE Study Extension, 2005). This drug simultaneously blocks the ACE-catalyzed degradation of kinins and the generation of angiotensin II, two tissue hormones that exhibit opposite biological or pharmacological effects.
A sense of tradition, combined with the concentration of research activities on the kallikrein-kinin system in Munich connected with other notable kinin centres worldwide, kept the memory of the two scientists from the University of Munich, Emil-Karl Frey and Eugen Werle, alive in the scientific community and has led to the establishment of the 'E.K. Frey-E. Werle Foundation of the Henning L. Voigt Family' in 1988.
References
Frey, E.K. (1926): Zusammenhänge zwischen Herzarbeit und Nierentätigkeit. Arch. Klin. Chir. 142, 663 - 669.
Frey, E.K. and Kraut, H. (1926): Über einen von der Niere ausgeschiedenen, die Herztätigkeit anregenden Stoff. Hoppe-Seyler's Z. Physiol. Chem. 157, 32 - 61.
Frey, E.K, Kraut, H. and Werle, E., eds. (1950): Kallikrein-Padutin. F. Enke-Verlag, Stuttgart, Germany.
Kraut, H., Frey, E.K. and Werle, E. (1930): Über die Inaktivierung des Kallikreins. Hoppe-Seyler' s Z. Physiol. Chem. 192, 1 - 21.
Werle, E., Götze, W., Kappler, A. (1937): Über die Wirkung des Kallikreins auf den isolierten Darm und über eine neue darmkontrahierende Substanz. Biochem. Z. 289, 217 - 233.
Werle, E. and Grunz, M. (1939): Zur Kenntnis der darmkontrahierenden, uterus-erregenden und blutdrucksenkenden Substanz DK. Biochem. Z. 301, 429 - 436.
The HOPE Study Investigators (1996): The HOPE (Heart Outcomes Prevention Evaluation) Study: the design of a large, simple randomized trial of an angiotensin-converting enzyme inhibitor (ramipril) and vitamin E in patients at high risk of cardiovascular events. Can J. Cardiol. 12, 127-137.
The Heart Outcomes Prevention Evaluation Study Investigators (2000): Vitamin E supplementation and cardiovascular events in high-risk patients. New Engl. J. Med. 342, 154 - 160.
HOPE/HOPE-TOO Study Investigators (2005): Results of the HOPE Study Extension: Long-term effects of ramipril on cardiovascular events and on diabetes. Circulation 112, 1339-1346.
History of the Origin and Rise of the Foundation
As a cardiologist who mastered catheterization procedures and as a specialist in endocrinology and metabolism research, Dietze was able to develop a methodology to measure substrate utilization in skeletal muscle, liver, and brain of human subjects. This approach ensured the safety of the subject and obtained the necessary information for the overall analysis and application of the metabolic results. With these studies it was possible for the first time to test the translatability of results from animal model-based research experiments to investigations involving humans, and therefore this line of investigation was of extreme importance not only for the Diabetes Research Institute, but also for the Special DFG Research Centre 51 in Munich. In addition to the Munich research group, at this point in time there were only three other groups in the world that had mastered this technique: George Cahill in Boston, Olly Owen in Philadelphia, and John Wahren in Stockholm.
When Emil-Karl Frey and Eugen Werle, two eminent faculty members at the medical school of the University of Munich, heard about this new technique, they encouraged Dietze to test the effect of bradykinin (BK) on the substrate balance in skeletal muscle, as they had observed in 1928 the blood pressure - and blood glucose - lowering effects of the enzyme kallikrein, which liberates BK from kininogen, in pancreatectomized dogs. Dietze and colleagues demonstrated that BK enhances blood flow and glucose uptake in skeletal muscle (Dietze G. & Wicklmayr M., Klin. Wochenschr. 55, 1977), and that BK is produced in contracting skeletal muscle (Wicklmayr et al., Horm. Metab. Res. 20, 1988) and stimulates a contraction-dependent increase in blood flow and glucose uptake in this tissue (Dietze G. & Wicklmayr M., FEBS Lett. 74, 1977). It was subsequently established that BK mediates its effects via its own receptors located both in the capillary endothelium and in the sarcolemma of the skeletal muscle cells (Figueroa C. D., Dietze G., Müller-Esterl W., Diabetes 45, 1996).

Guenther Dietze
In 1970, Ervin Erdös discovered that the actions of the KKS and the RAS were linked and counterregulatory (Yang H. Y. & Erdös E.G., Biochim. Biophys. Acta 214, 1970). He showed that ACE not only catalyzes the conversion of ANG I to ANG II, but also causes the simultaneous inactivation of BK. This represented the discovery of a new biochemical mechanism of great physiological importance, and subsequent work from Dietze's research group in Munich and the research group of Prof. Erik J. Henriksen in the Department of Physiology at the University of Arizona, among others, indicated a central role of the RAS in the regulation of blood pressure and glucose homeostasis (Dietze G. et al. in: Hemodynamics of the Limbs, Puel P. et al. Eds., Plenum Press, New York-London, 1979; Dietze G. J. & Henriksen E. J., JRAAS 9, 2008).
The Munich research group led by Prof. Dr. Dietze also showed that subjects with type 2 diabetes and with hypertension, who display insulin resistance, release significantly less BK from skeletal muscle during muscle contractions (Wicklmayr M. et al., Horm. Metab. Res. 21, 1989). Both the diminished capacity for contraction-stimulated BK release and the insulin resistance could be ameliorated by treatment with an ACE inhibitor (such as captopril) (Henriksen EJ et al., Diabetes 45 1996), providing furher support that the RAS represents an important target in the underlying pathophysiology of hypertension and insulin resistance in humans.
The RAS represents a critical aspect of all risk factors for the development of atherosclerosis: male gender, aging, inheritance of the ACE-D allele, stress, overweight and obesity, chronic tobacco use, high blood pressure, insulin resistance and type 2 diabetes, and elevated blood cholesterol and triglycerides (Dietze G. J. & Henriksen E. J., JRAAS 9, 2008). Numerous international scientific symposia, which in part were planned in cooperation with the U. S. National Institutes of Health, have addressed this subject, and the resulting compendia have been published as supplements in Hormone and Metabolic Research (1990), Diabetes (1996), and the American Journal of Cardiology (1997 and 1998).
The many scientific contributions that have clarified the importance of the two counterbalancing systems - the KKS and the RAS - in the physiological regulation of blood pressure and glucose homeostasis and in the pathophysiological development of insulin resistance/type 2 diabetes, hypertension, and subsequently atherosclerosis in humans inspired Senator Henning L. Voigt to recognize the importance of the Munich research group in this context and to establish the "E. K. Frey-E. Werle Foundation" of the University of Munich in 1988.
Guenther J. Dietze 2017 (English translation by Erik J. Henriksen)
25 Years Welfare Engagement for the FREY-WERLE Foundation
After the Study of Chemistry at the Technical Universitiy Munich,
completed by Diploma (1961) and PhD/Dr. rer. nat (1963), Hans Fritz
joined Eugen Werle at the Medical Faculty of LMU Munich and was
nominated University Lecturer MD habil. there 1968. Thereafter, he was
appointed Extraordinary Professor at the Medical Faculty of LMU (1974)
and 1978 Full Professor of Clinical Chemistry and Head of the Division
of Clinical Chemistry and Clinical Biochemistry at the Department of
Surgery Downtown at the LMU until 2001.
In 1974 Hans Fritz received the Carl Duisberg Award for outstanding
scientific work in Medicinal Chemistry, and was appointed 1985
Associate Member of the Institute Jozef Stefan in Ljubljana/Slovenia.
He chaired the Special Research Centres SFB 51 and SFB 207 of LMU
Munich from 1978-1996 and became also Governor of the HENNER GRAEFF
Foundation 2013.
Farewell to Hans Fritz
Our highly esteemed Executive Editor, Professor Hans Fritz, Ludwig-Maximilians-University Munich, is finishing his service with Biological Chemistry in this function, to be succeeded by Professor Boris Turk (see Editors’ Note on page 963). We look forward to his continued contribution as a member of the Editorial Board. Hans Fritz was instrumental in shaping the journal to its present level of international recognition for several decades, and with his expertise on proteolysis he made this area of research a mainstay of the journal. He served as Executive Editor since 1993, and prior to this he had been associated with the journal as a member of the Editorial Board. Equally important, of the 420 research articles authored by Hans Fritz, 106 have been published in this journal, previously known as Hoppe-Seyler’s Zeitschrift für Physiologische Chemie. Hans came to the University of Munich in 1963 and joined the group of Eugen Werle, beginning his research on protease inhibitors, many of which he first described in the literature. Early on, he was active in organizing meetings, and the first international conference on protease inhibitors was published in 1971: ‘Proceedings of the International Conference on Proteinase Inhibitors, Munich, Nov. 1970’, H. Fritz and H. Tschesche (eds.), Walter de Gruyter, Berlin-New York, 1971. Further work focused on proteases (e.g., sperm acrosin, leukocyte elastase) and the kallikrein-kinin system. His research grant support started in 1969 in the Sonderforschungsbereich (SFB) 51 of the Deutsche Forschungsgemeinschaft (DFG), with Theodor Bücher and Feodor Lynen, project leaders including Robert Huber.

Hans Fritz
Helmut Sies, Editor-in-Chief
Biol. Chem. 389, p. 965 (2008)
